Recommendations for Intraoperative Adverse Events Data Collection in Clinical Studies and Study Protocols. An ICARUS Global Surgical Collaboration Study. - Abstract - Europe PMC
Por um escritor misterioso
Last updated 17 junho 2024

Europe PMC is an archive of life sciences journal literature.
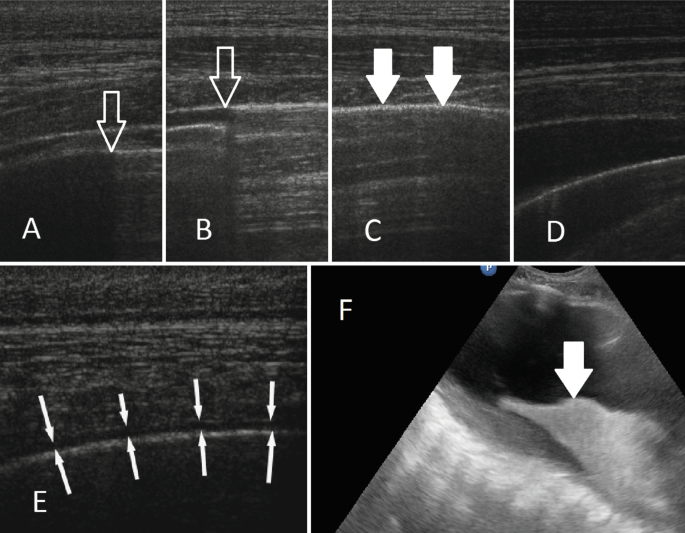
Diagnostic Imaging in Space Medicine

The Comprehensive Complication Index. Proposed modification to improve estimates of perioperative morbidity after radical cystectomy. A pilot study. - Abstract - Europe PMC

Tocilizumab administration for the treatment of hospitalized patients with COVID‐19: A systematic review and meta‐analysis - Kyriakopoulos - 2021 - Respirology - Wiley Online Library
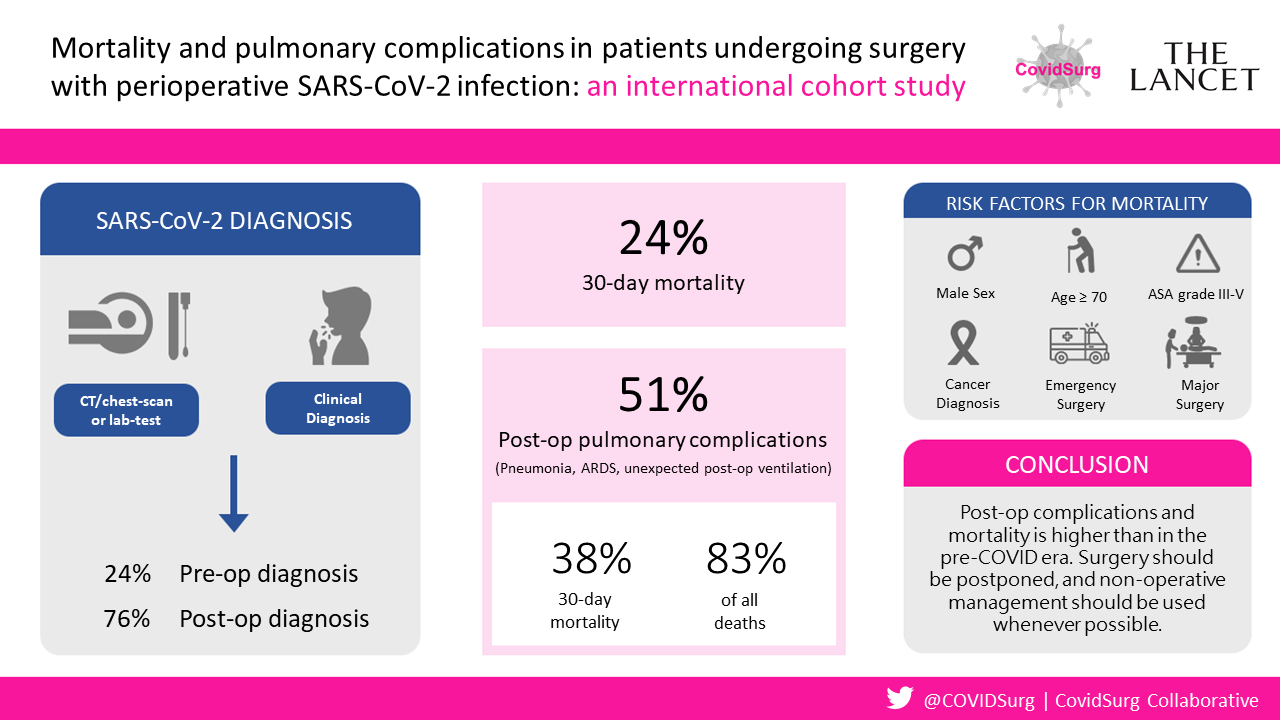
CovidSurg Collaborative article in The Lancet – Globalsurg

Words 333333, PDF, Internet

Visual Abstracts: Innovations: Sage Journals

PDF] A Protocol for the Development of the Intraoperative Complications Assessment and Reporting With Universal Standards Criteria: The ICARUS Project

Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study - The Lancet

Cytoreductive surgery and intraperitoneal chemotherapy: an evidence-based review-past, present and future. - Abstract - Europe PMC

Recommendations for Intraoperative Adverse Events Data Collection in Clinical Studies and Study Protocols. An ICARUS Global Surgical Collaboration Study. - Abstract - Europe PMC

PDF) Recommendations for Intraoperative Adverse Events Data Collection in Clinical Studies and Study Protocols. An ICARUS Global Surgical Collaboration Study
Recomendado para você
-
 Jacob Barata Filho recebeu informação da Caruana sobre quebra de sigilo e quase fugiu, apontam documentos do MPF17 junho 2024
Jacob Barata Filho recebeu informação da Caruana sobre quebra de sigilo e quase fugiu, apontam documentos do MPF17 junho 2024 -
 File:Caruana, Jaime (IMF 2008) (frame).jpg - Wikimedia Commons17 junho 2024
File:Caruana, Jaime (IMF 2008) (frame).jpg - Wikimedia Commons17 junho 2024 -
 Resumen - Universidad de Navarra17 junho 2024
Resumen - Universidad de Navarra17 junho 2024 -
 How can I fix this bug? : r/Anki17 junho 2024
How can I fix this bug? : r/Anki17 junho 2024 -
 Eurozone Crisis and Banks' Creditworthiness: What is New for Credit Default Swap Spread Determinants? - Alessandra Ortolano, Eliana Angelini, 202217 junho 2024
Eurozone Crisis and Banks' Creditworthiness: What is New for Credit Default Swap Spread Determinants? - Alessandra Ortolano, Eliana Angelini, 202217 junho 2024 -
 Flashback pluggable database no Data Guard17 junho 2024
Flashback pluggable database no Data Guard17 junho 2024 -
Full article: The impact of credit shocks on the European labour market17 junho 2024
-
 Just found my second and third cake soul, neat. : r/HypixelSkyblock17 junho 2024
Just found my second and third cake soul, neat. : r/HypixelSkyblock17 junho 2024 -
 DataCamp or Google Data Analysis on Coursera? : r/DataCamp17 junho 2024
DataCamp or Google Data Analysis on Coursera? : r/DataCamp17 junho 2024 -
Brazil Lending Rate: per Annum: Pre-Fixed: Individuals: Overdraft: Banco A. J. Renner S.A., Economic Indicators17 junho 2024
você pode gostar
-
Phillip Chu Joy - GG WP Durante nuestras partidas multijugador siempre habrán momentos donde podamos tener ventaja sobre nuestros oponentes, donde tengamos la estrategia ya hecha, donde sepamos que todo está ganado17 junho 2024
-
 My wife kept saying that Henry Cavill is a Greek God so I made him17 junho 2024
My wife kept saying that Henry Cavill is a Greek God so I made him17 junho 2024 -
 Need For Speed Movie, Cast and crew from the up coming movi…17 junho 2024
Need For Speed Movie, Cast and crew from the up coming movi…17 junho 2024 -
 Against the Storm (Original Game Soundtrack)17 junho 2024
Against the Storm (Original Game Soundtrack)17 junho 2024 -
 FREE! - Atividade de Matemática com multiplicação – Twinkl17 junho 2024
FREE! - Atividade de Matemática com multiplicação – Twinkl17 junho 2024 -
 8.962.988 fotos de stock e banco de imagens de Jogador De Basquete - Getty Images17 junho 2024
8.962.988 fotos de stock e banco de imagens de Jogador De Basquete - Getty Images17 junho 2024 -
 Cristiano Ronaldo rows with cameraman after being subbed17 junho 2024
Cristiano Ronaldo rows with cameraman after being subbed17 junho 2024 -
Marvel Snap Tier ListDecember 2023 – The Best Decks-Game Guides17 junho 2024
-
 Animes Vision on X: Episódios 15, 16 e 17 de Kimetsu no Yaiba foram trocados pela versão blu-ray, e já estão disponíveis para streaming e download! / X17 junho 2024
Animes Vision on X: Episódios 15, 16 e 17 de Kimetsu no Yaiba foram trocados pela versão blu-ray, e já estão disponíveis para streaming e download! / X17 junho 2024 -
 Erased - Kayo Hinazuki walking in the snow, titled Hinazuki Kayo17 junho 2024
Erased - Kayo Hinazuki walking in the snow, titled Hinazuki Kayo17 junho 2024
