FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 15 junho 2024

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
FDA issues emergency use authorization for bebtelovimab - The

FDA announces Evusheld is not currently authorized for emergency

US allows 1st emergency use of a COVID-19 antibody drug

FDA won't comment on status of Emergency Use Authorizations for

Federal Register :: Authorizations of Emergency Use of Certain
FDA Meeting on COVID Vaccine for Children
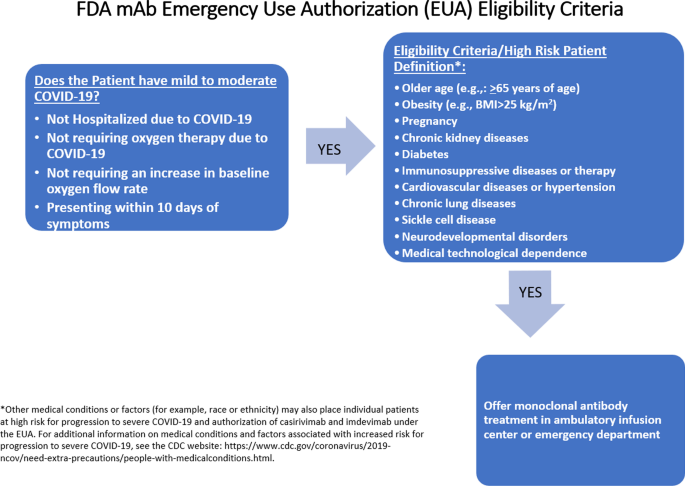
Assessment of unvaccinated and vaccinated patients with

Federal Register :: Authorizations of Emergency Use of Certain

Moderna asks the F.D.A. to authorize its vaccine for children

Federal Register :: Authorizations of Emergency Use of Certain
:max_bytes(150000):strip_icc()/GettyImages-1316976332-546104aabbac456eb451bc98e18f75c8.jpg)
FDA Revokes Authorization for the Only Remaining COVID-19
Recomendado para você
-
 Dr. Isaac Azar, MD – Aventura, FL15 junho 2024
Dr. Isaac Azar, MD – Aventura, FL15 junho 2024 -
 Histórias e raízes de Angelita e Isaac15 junho 2024
Histórias e raízes de Angelita e Isaac15 junho 2024 -
 Isaac Azar, MD, Aventura, FL Emergency Medicine Physician15 junho 2024
Isaac Azar, MD, Aventura, FL Emergency Medicine Physician15 junho 2024 -
 Dr. Isaac Dario Azar MD, Emergency Physician in AVENTURA, FL, 3318015 junho 2024
Dr. Isaac Dario Azar MD, Emergency Physician in AVENTURA, FL, 3318015 junho 2024 -
 Our Team Advanced Implant Educators15 junho 2024
Our Team Advanced Implant Educators15 junho 2024 -
 Jose DIAZ, Chief Acute Care Surgery, University of South Florida, FL, USF, Department of Surgery15 junho 2024
Jose DIAZ, Chief Acute Care Surgery, University of South Florida, FL, USF, Department of Surgery15 junho 2024 -
 David Leverenz Duke Department of Medicine15 junho 2024
David Leverenz Duke Department of Medicine15 junho 2024 -
Gary G. Bennett Office of Undergraduate Education15 junho 2024
-
Health Care Policy Priorities for 117th Congress15 junho 2024
-
 Announcing Our November Book Club Selection: Home Reading Service by Fabio Morábito - Asymptote Blog15 junho 2024
Announcing Our November Book Club Selection: Home Reading Service by Fabio Morábito - Asymptote Blog15 junho 2024
você pode gostar
-
 General Renmus, Elder Scrolls15 junho 2024
General Renmus, Elder Scrolls15 junho 2024 -
 Passe delicioso de Gvardiol e Foden assina o empate para o City15 junho 2024
Passe delicioso de Gvardiol e Foden assina o empate para o City15 junho 2024 -
 Otaku Nuts: Assassination Classroom Anime Review15 junho 2024
Otaku Nuts: Assassination Classroom Anime Review15 junho 2024 -
 Five Night Freddy Xbox 36015 junho 2024
Five Night Freddy Xbox 36015 junho 2024 -
 Dragon Ball Super - Fierce Battle Against A Might Foe15 junho 2024
Dragon Ball Super - Fierce Battle Against A Might Foe15 junho 2024 -
 Dragon Ball Z15 junho 2024
Dragon Ball Z15 junho 2024 -
 Pintura por números pintando por números boné à noite, 40x60 cm, quadro ra236 pintura de desenho15 junho 2024
Pintura por números pintando por números boné à noite, 40x60 cm, quadro ra236 pintura de desenho15 junho 2024 -
 Link (Ocarina of Time), Versus Compendium Wiki15 junho 2024
Link (Ocarina of Time), Versus Compendium Wiki15 junho 2024 -
ousama ranking ep 10|TikTok Search15 junho 2024
-
 When is Thanksgiving 2021?15 junho 2024
When is Thanksgiving 2021?15 junho 2024
