FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Last updated 16 junho 2024

Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed
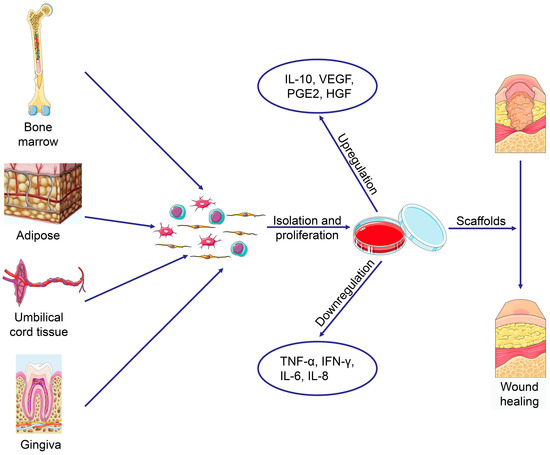
Cells, Free Full-Text
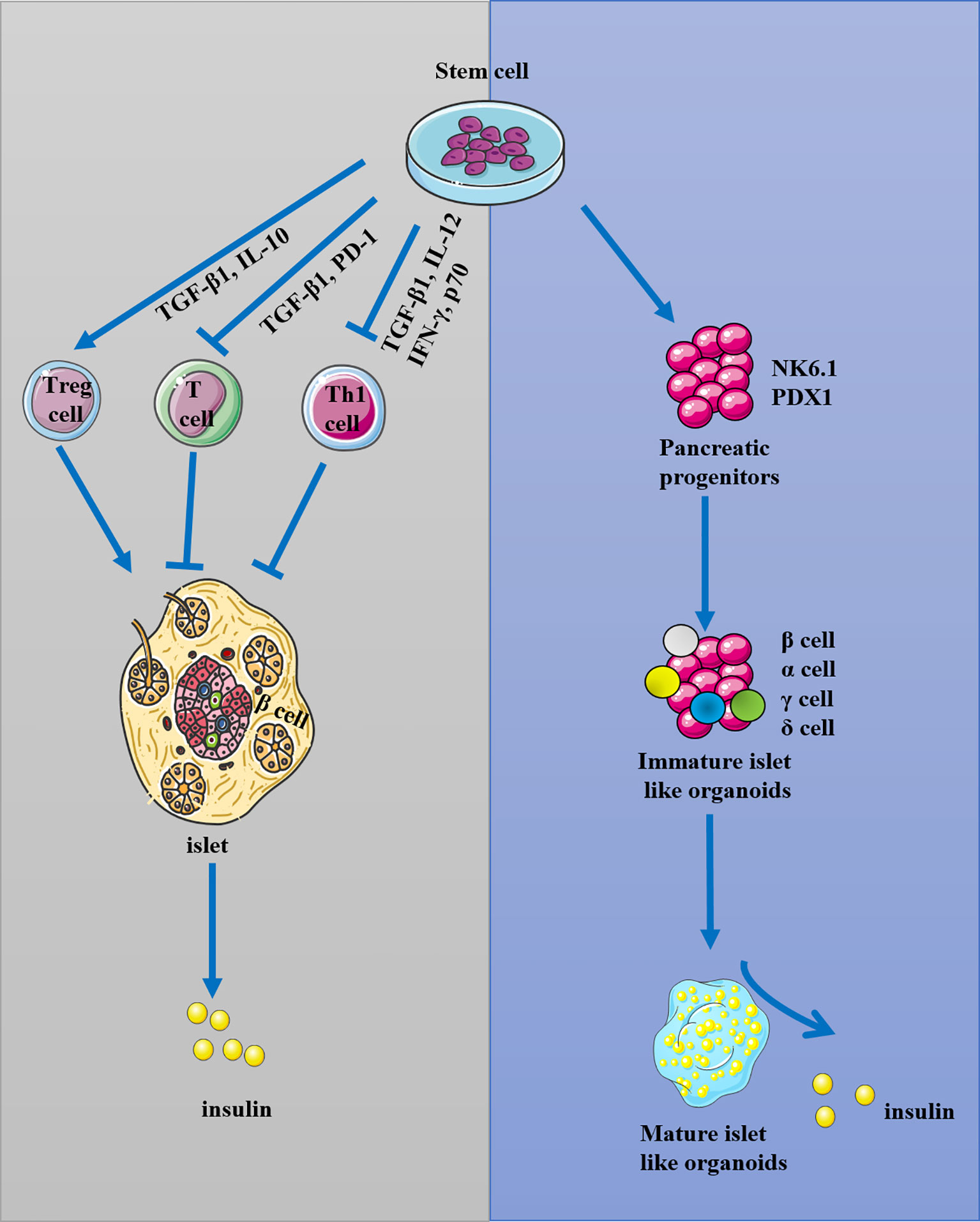
Frontiers Stem Cell Transplantation in the Treatment of Type 1

FDA Approves Posttransplant Cell Therapy for Patients with Blood

Early Trial of Orca-T Produces 100% OS Rate in Hematologic

A Judge Rules Against One Stem-Cell Clinic. There Are Hundreds of

Systematic Review of Induced Pluripotent Stem Cell Technology as a

Weekly reads: FDA nod on new cell therapy, gray hair, pong-playing

Despite Progress, Government Can Better Protect Patients From

FDA approves cell therapy for patients with blood cancers to

Adult Acute Lymphoblastic Leukemia Treatment (PDQ®) - PDQ Cancer
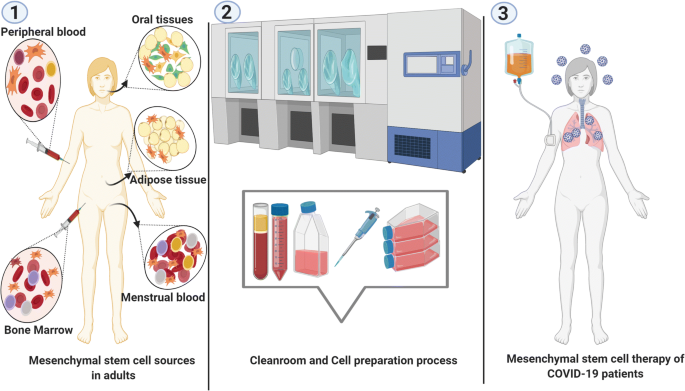
Cell-Based Therapy for Severe COVID-19 Patients: Clinical Trials
Recomendado para você
-
 English Today Cambridge Core16 junho 2024
English Today Cambridge Core16 junho 2024 -
George Clay Fire Company - Station 39, West Conshohocken PA16 junho 2024
-
 Thiago Silva is 39 today - Our Brazilian legend is the finest of wines 💙 : r/chelseafc16 junho 2024
Thiago Silva is 39 today - Our Brazilian legend is the finest of wines 💙 : r/chelseafc16 junho 2024 -
 Egypt receives lists for 13 Israelis and 39 Palestinians for release today16 junho 2024
Egypt receives lists for 13 Israelis and 39 Palestinians for release today16 junho 2024 -
/cdn.vox-cdn.com/uploads/chorus_asset/file/24909597/usa_today_21335839.jpg) 2023 MAC Football Week 8 Game Recap: Toledo Rockets 21, Miami RedHawks 17 - Hustle Belt16 junho 2024
2023 MAC Football Week 8 Game Recap: Toledo Rockets 21, Miami RedHawks 17 - Hustle Belt16 junho 2024 -
 Winter 2023 - Florida CPA Today Volume 39, Issue 1 by Florida Institute of CPAs - Issuu16 junho 2024
Winter 2023 - Florida CPA Today Volume 39, Issue 1 by Florida Institute of CPAs - Issuu16 junho 2024 -
 Aos 39 anos, zagueiro ex-seleção inglesa é dispensado na segunda divisão do país16 junho 2024
Aos 39 anos, zagueiro ex-seleção inglesa é dispensado na segunda divisão do país16 junho 2024 -
 Global Cryptocurrency Owners Grow to 425 million through 202216 junho 2024
Global Cryptocurrency Owners Grow to 425 million through 202216 junho 2024 -
 How to get the Philadelphia Eagles Nike Pegasus 39 sneakers today16 junho 2024
How to get the Philadelphia Eagles Nike Pegasus 39 sneakers today16 junho 2024 -
 39 Problems, bar and restaurant in Albion, reopens on Main Street16 junho 2024
39 Problems, bar and restaurant in Albion, reopens on Main Street16 junho 2024
você pode gostar
-
 Rukh no Teikoku – Problematizando o mundo dos animes. Reviews16 junho 2024
Rukh no Teikoku – Problematizando o mundo dos animes. Reviews16 junho 2024 -
 The Amazing Spider-Man Venom Ben Reilly Spider-Man 2099, spider-man, heroes, superhero png16 junho 2024
The Amazing Spider-Man Venom Ben Reilly Spider-Man 2099, spider-man, heroes, superhero png16 junho 2024 -
 ARTE PARA PINTAR - CONSCIÊNCIA NEGRA16 junho 2024
ARTE PARA PINTAR - CONSCIÊNCIA NEGRA16 junho 2024 -
 Terraria: Armor Tier List 1.4.4 - Item Level Gaming16 junho 2024
Terraria: Armor Tier List 1.4.4 - Item Level Gaming16 junho 2024 -
 Kids - Minecraft Nintendo Switch, Skins, Unblocked, Mods, Download, Servers, Achievements, Wiki, Maps, APK, Game Guide Unofficial - Dayton Metro Library - OverDrive16 junho 2024
Kids - Minecraft Nintendo Switch, Skins, Unblocked, Mods, Download, Servers, Achievements, Wiki, Maps, APK, Game Guide Unofficial - Dayton Metro Library - OverDrive16 junho 2024 -
 Roblox Noob v1 for GTA San Andreas16 junho 2024
Roblox Noob v1 for GTA San Andreas16 junho 2024 -
 Read Record Of Ragnarok Manga on Mangakakalot16 junho 2024
Read Record Of Ragnarok Manga on Mangakakalot16 junho 2024 -
 Swan Fridays - World Red Eye16 junho 2024
Swan Fridays - World Red Eye16 junho 2024 -
 Gran Turismo 7: Anniversary Edition vs Standard?16 junho 2024
Gran Turismo 7: Anniversary Edition vs Standard?16 junho 2024 -
Matemática Gis com Giz - Saiu o vídeo do SALVE! Corre lá no canal da Gis! 😊16 junho 2024

